Acetyl salicylic acid commonly known as Aspirin. It is prepared by the reaction of salicylic acid and acetic anhydride (as shown below) . 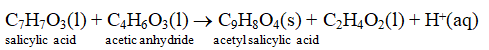
If 10.0 ml of salicylic acid (1.443 g/mL) reacts with 15 mL of acetic anhydride (1.082 g/mL) , then how many moles of acetyl salicylic acid would be produced?
A) 0.160 moles
B) 0.104 moles
C) 1.022 moles
D) 0.456 moles
E) 0.136 moles
Correct Answer:
Verified
Q52: In a balanced chemical reaction the number
Q53: One mole of NH4OH will react with
Q54: A(n) _is the amount of product or
Q55: _ is the maximum amount of product(s)
Q56: The oxidation state of a copper atom
Q58: When a metal is oxidized it loses
Q59: To balance the equation given below, the
Q60: Elaborate on the steps followed during the
Q61: The composition of the atmosphere is constant
Q62: Molecular fluorine is a very powerful reducing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents