What value of Z (atomic number) and A (mass number) result in the following gamma decay? 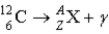
A) Z = 5; A = 12
B) Z = 4; A = 8
C) Z = 7; A = 12
D) Z = 6; A = 12
E) Z = 6; A = 11
Correct Answer:
Verified
Q24: In beta decays
A) a proton changes to
Q25: When a neutron decays, a proton and
Q26: The Q value for the following reaction,
Q27: Heavy nuclei are unstable because
A) each nucleon
Q28: Which of the effects listed below is
Q30: In nuclear magnetic resonance, nuclei absorb energy
Q31: The chart below shows part of the
Q32: What is the disintegration energy (in MeV)
Q33: Because we know that the half-lives of
Q34: Two nuclei which share the same atomic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents