If P(r) is the radial probability density function for an electron in the ground state of a hydrogen atom, the most probable value for r can be found from
A) dP/dt
B) dP/dr
C) 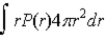
D) 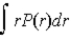
E) d2P/dr2
Correct Answer:
Verified
Q26: When using the Pauli Exclusion Principle, we
Q27: The radial portion of the de Broglie
Q28: A Li2+ ion undergoes a transition from
Q29: The probability density for the 1s state
Q30: Rubidium (Z = 37) and potassium (Z
Q32: The probability density of a particle at
Q33: The magnitude of the spin angular momentum
Q34: Forbidden transitions and selection rules suggest that
A)
Q35: When electrons fill a subshell in which
Q36: For the following allowed transitions, which photon
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents