If n moles of an ideal gas are compressed isothermally from an initial volume V1 to a final volume V2, the change in entropy is
A) nR ln (V2/V1)
B) nRT ln (V2/V1)
C) nkB ln (V2/V1)
D) 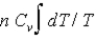
E) n Cv/T
Correct Answer:
Verified
Q27: The change in entropy of a mass
Q28: The reason that we can calculate the
Q29: An ideal heat engine can have an
Q30: An adiabatic free expansion of a gas
Q31: Ten kilograms of water at 0°C is
Q33: Since Lice = 333 J/g, the change
Q34: Determine the change in entropy (in J/K)
Q35: A Carnot cycle, operating as a heat
Q36: Find the change in entropy (in J/K)
Q37: A vessel containing 10 kg of water
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents