The average molecular translational kinetic energy of a molecule in an ideal gas is
A) 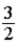 kBT.
kBT.
B) 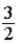 RT.
RT.
C) 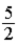 kBT.
kBT.
D) 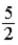 RT.
RT.
E) 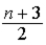 kBT, where n = number of internal degrees of freedom.
kBT, where n = number of internal degrees of freedom.
Correct Answer:
Verified
Q1: When we say that the speed of
Q2: A container having a volume of 1.0
Q3: An ideal gas is allowed to expand
Q4: The theorem of equipartition of energy states
Q6: The average translational speed of a nitrogen
Q7: Suppose a box contains about 5.0 ×
Q8: Five gas molecules are found to have
Q9: A container having a volume of 1.0
Q10: Assume 3.0 moles of a diatomic gas
Q11: Which statement below is NOT an assumption
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents