The relation PV = nRT holds for all ideal gases. The additional relation PVγ holds for an adiabatic process. The figure below shows two curves: one is an adiabat and one is an isotherm. Each starts at the same pressure and volume. Which statement is correct? (Note: "∝" means "is proportional to".) 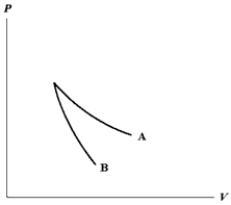
A) Isotherm: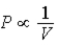 ; Adiabat:
; Adiabat: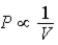 : A is both an isotherm and an adiabat.
: A is both an isotherm and an adiabat.
B) Isotherm: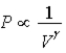 ; Adiabat:
; Adiabat: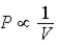 : B is an isotherm, A is an adiabat.
: B is an isotherm, A is an adiabat.
C) Isotherm: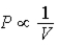 ; Adiabat:
; Adiabat: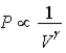 : A is an isotherm, B is an adiabat.
: A is an isotherm, B is an adiabat.
D) Isotherm: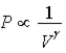 ; Adiabat:
; Adiabat: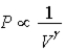 : B is both an isotherm and an adiabat.
: B is both an isotherm and an adiabat.
E) cannot answer without additional information about the starting temperature.
Correct Answer:
Verified
Q8: Five gas molecules are found to have
Q9: A container having a volume of 1.0
Q10: Assume 3.0 moles of a diatomic gas
Q11: Which statement below is NOT an assumption
Q12: The air in an automobile engine at
Q14: The average kinetic energy of a nitrogen
Q15: The internal energy of n moles of
Q16: Nitrogen gas is heated by a pulsed
Q17: Find the specific heat (in cal/mole K)
Q18: A molecule in a uniform ideal gas
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents