The molar specific heat at constant pressure at 0°C of an ideal monatomic gas is
A) 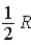 .
.
B) R.
C) 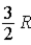 .
.
D) 2R.
E) 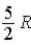 .
.
Correct Answer:
Verified
Q19: Assume molecules have an average diameter of
Q20: During an adiabatic compression, a volume of
Q21: The temperature of a quantity of an
Q22: The molar specific heat at constant volume
Q23: One mole of hydrogen, one mole of
Q25: The specific heat of an ideal gas
Q26: One mole of helium gas expands adiabatically
Q27: If the total translational kinetic energy of
Q28: If the rms speed of helium atoms
Q29: Two tanks of gas, one of hydrogen,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents