Nuclear binding energy: The neutral deuterium atom,  H, has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. What is the binding energy of the
H, has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. What is the binding energy of the 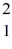 H nucleus? (1 u = 931.494 MeV/c2)
H nucleus? (1 u = 931.494 MeV/c2)
A) 1.1 MeV
B) 1.7 MeV
C) 2.2 MeV
D) 2.9 MeV
E) 3.4 MeV
Correct Answer:
Verified
Q19: Properties of the nucleus: Which of the
Q20: Radioactive decay: A radioactive isotope decays by
Q21: Nuclear binding energy: Uranium-238 decays into thorium-234
Q22: Nuclear fusion: A fusion reaction releases energy
Q23: Nuclear fusion: The primary source of the
Q25: Nuclear binding energy: The carbon in your
Q26: Nuclear binding energy: The set of nuclear
Q27: Nuclear binding energy: A stationary plutonium-239 nucleus
Q28: Nuclear fusion: How does the mass of
Q29: Properties of the nucleus: A certain nucleus
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents