Radioactivity: The stability of 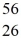 Fe with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known:
Fe with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known: 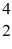 He: 4.002603 u
He: 4.002603 u 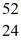 Cr: 51.944768 u
Cr: 51.944768 u 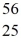 Mn: 55.938907 u
Mn: 55.938907 u 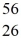 Fe: 55.934939 u
Fe: 55.934939 u 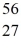 Co: 55.939841 u The
Co: 55.939841 u The 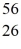 Fe nuclide is
Fe nuclide is
A) not subject to alpha, β+, or β- decay.
B) subject to alpha decay only.
C) subject to β+decay only.
D) subject to β- decay only.
E) subject to β+ or β- decay, but not to alpha decay.
Correct Answer:
Verified
Q36: Properties of the nucleus: What would be
Q37: Properties of the nucleus: Two identical nuclei
Q38: Nuclear binding energy: Plutonium-239 decays into uranium-235
Q39: Properties of the nucleus: A certain nucleus
Q40: Nuclear binding energy: How much energy is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents