Radioactive decay: What mass of 14C (having a half-life of 5730 years) do you need to provide a decay rate of 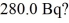 (1 u = 1.6605 × 10-27 kg)
(1 u = 1.6605 × 10-27 kg)
A) 1.70 × 10-12 kg
B) 5.38 × 10-19 kg
C) 3.84 × 10-20 kg
D) 8.68 × 10-13 kg
Correct Answer:
Verified
Q48: Radioactive decay: An air sample is contaminated
Q49: Radioactivity: A sphere made of a radioactive
Q50: Radioactive decay: In a laboratory accident a
Q51: Radioactive decay: A radioactive sample has a
Q52: Radioactive decay: A certain substance has a
Q54: Radioactive decay: An isotope of Tc having
Q55: Radioactive dating: Carbon-14 has a half-life of
Q56: Radioactive decay: A hospital patient has been
Q57: Radioactivity: The stability of Q58: Radioactive decay: The unstable isotope 234Th decays![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents