Nuclear fusion: Consider the fusion reaction 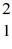 H +
H + 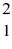 H +
H + 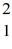 H →
H → 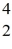 He +
He + 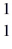 H +
H + 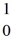 n
n
The atomic masses are: 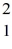 H: 2.01410 u
H: 2.01410 u 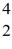 He: 4.00260 u
He: 4.00260 u 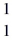 H, 1.00783 u
H, 1.00783 u 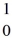 n: 1.008665 u
n: 1.008665 u
What mass of deuterium fuel, 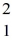 H, is used up in producing 8.2 × 1013 J of energy by this reaction? (1u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
H, is used up in producing 8.2 × 1013 J of energy by this reaction? (1u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
Correct Answer:
Verified
Q79: Nuclear fusion: Calculate the amount of energy
Q80: Reaction energy: Calculate the reaction energy (Q
Q81: Nuclear fusion: The reaction 2H + 2H
Q82: Biomedicine: The radioactive nuclei 60Co are widely
Q83: Biomedicine: A certain isotope has a half-life
Q84: Biomedicine: A 70-kg laboratory technician absorbs 2.9
Q85: Nuclear fusion: Two deuterium nuclei, 
Q86: Biomedicine: A 57-kg researcher absorbs 6.3 ×
Q88: Nuclear fusion: Two deuterium nuclei, 
Q89: Biomedicine: The maximum permissible workday dose for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents