Quantum numbers: In the ground state, the quantum numbers (n, l, ml, ms) for hydrogen are, respectively
A) 1, 1, 1, 1.
B) 1, 0, 0, 0.
C) 1, 0, 0, ± 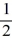 .
.
D) 1, 1, 1, ± 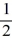 .
.
E) 1, 1, 0, ± 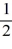
Correct Answer:
Verified
Q12: Hydrogen atom: What is the minimum speed
Q13: Zeeman effect: Electrons in the presence of
Q14: Quantum numbers: An electron in a hydrogen
Q15: Hydrogen atom: The normalized wave function for
Q16: Hydrogen atom: How fast must a hydrogen
Q18: Hydrogen atom: What is the energy of
Q19: Quantum numbers: An electron in a hydrogen
Q20: Quantum numbers: Consider the four quantum numbers
Q21: Hydrogen atom: What is the radius of
Q22: Multielectron atoms: The only VALID electron state
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents