Lasers: A collection of atoms has 20% of the sample in a state 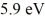 above the ground state. If these emit coherent radiation, what is the wavelength of the laser light produced? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
above the ground state. If these emit coherent radiation, what is the wavelength of the laser light produced? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
A) 210 nm
B) 91 nm
C) 340 nm
D) 34 nm
Correct Answer:
Verified
Q37: Angular momentum: What is the greatest magnitude
Q38: Angular momentum: What is the greatest total
Q39: Hydrogen atom: A muonic hydrogen atom is
Q40: Hydrogen atom: Doubly-ionized lithium is a hydrogen-like
Q41: Zeeman effect: The energy of an electron
Q43: Lasers: How many photons per second emerge
Q44: Zeeman effect: An s state (l =
Q45: Multielectron atoms: What is the correct electronic
Q46: Zeeman effect: An alkali metal atom is
Q47: Zeeman effect: An atom in a state
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents