Particle in a box: An electron is in an infinite square well (a box) that is 2.0 nm wide. The electron makes a transition from the  to the
to the 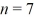 state, what is the wavelength of the emitted photon? (h = 6.626 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg, 1 eV = 1.60 × 10-19 J)
state, what is the wavelength of the emitted photon? (h = 6.626 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg, 1 eV = 1.60 × 10-19 J)
A) 880 nm
B) 750 nm
C) 610 nm
D) 1000 nm
E) 1100 nm
Correct Answer:
Verified
Q1: Normalization: The wave function for a particle
Q2: Particle in a box: The smallest kinetic
Q4: Particle in a box: The ground state
Q5: Harmonic oscillator: If an atom in a
Q6: Particle in a box: A particle is
Q7: Normalization: Find the value of A to
Q8: Particle in a box: An electron is
Q9: Normalization: A set of five possible wave
Q10: Particle in a box: A particle is
Q11: Normalization: Find the value of A to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents