Bohr atom: A hydrogen atom is in its n = 2 excited state when its electron absorbs a photon of energy 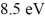 . What is the energy of the resulting free electron? The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
. What is the energy of the resulting free electron? The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
A) 5.1 eV
B) 6.6 eV
C) 6.9 eV
D) 7.7 eV
Correct Answer:
Verified
Q25: Blackbody radiation: In the vicinity of what
Q26: Compton scattering: In a particular case of
Q27: Bohr atom: The energy of the ground
Q28: Bohr atom: Suppose that in a parallel
Q29: Blackbody radiation: What is the wavelength of
Q31: Bohr atom: The energy of the ground
Q32: Bohr atom: A hydrogen atom initially in
Q33: Bohr atom: A hydrogen atom makes a
Q34: Bohr atom: Light shines through atomic hydrogen
Q35: Compton scattering: A beam of X-rays at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents