Quantity of heat: A 648-g empty iron kettle is put on a stove. How much heat. in joules. must it absorb to raise its temperature from 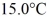 to
to 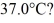 (The specific heat for iron is 113 cal/kg ∙ °C, 1 cal = 4.190 J)
(The specific heat for iron is 113 cal/kg ∙ °C, 1 cal = 4.190 J)
A) 6740 J
B) 11,300 J
C) 1610 J
D) 16,100 J
Correct Answer:
Verified
Q29: Quantity of heat: If we use 67
Q30: Thermal expansion: The coefficient of volume expansion
Q31: Thermal expansion: You want to insert an
Q32: Thermal expansion: A rod has a length
Q33: Phase changes: Heat is added to a
Q35: Thermal expansion: The coefficient of linear expansion
Q36: Thermal expansion: A glass flask has a
Q37: Thermal expansion: The exterior of a supersonic
Q38: Molecular speeds: A container is filled with
Q39: Quantity of heat: It is necessary to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents