Phase changes: Heat is added to a 2.0 kg piece of ice at a rate of 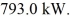 How long will it take for the ice to melt if it was initially at 0.00°C? (The latent heat of fusion for water is 334 kJ/kg and its latent heat of vaporization is 2260 kJ/kg.)
How long will it take for the ice to melt if it was initially at 0.00°C? (The latent heat of fusion for water is 334 kJ/kg and its latent heat of vaporization is 2260 kJ/kg.)
A) 0.84 s
B) 530,000 s
C) 4.7 s
D) 670 s
Correct Answer:
Verified
Q47: Calorimetry: Two experimental runs are performed to
Q48: Conduction of heat: Under steady state conditions,
Q49: Phase changes: If 2.0 g of water
Q50: Calorimetry: A person pours 330 g of
Q51: Calorimetry: An 80-g aluminum calorimeter contains 380
Q53: Conduction of heat: A solid concrete wall
Q54: Conduction of heat: Two metal rods, one
Q55: Calorimetry: A block of ice at 0.000°C
Q56: Calorimetry: A person makes ice tea by
Q57: Phase changes: A 406.0 kg copper bar
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents