Calorimetry: A 200-g metal container, insulated on the outside, holds 100 g of water in thermal equilibrium at 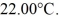 A 21-g ice cube, at the melting point, is dropped into the water, and when thermal equilibrium is reached the temperature is 15.00°C. Assume there is no heat exchange with the surroundings. For water, the specific heat is 4190 J/kg · K and the heat of fusion is 3.34 × 105 J/kg. The specific heat for the metal is closest to
A 21-g ice cube, at the melting point, is dropped into the water, and when thermal equilibrium is reached the temperature is 15.00°C. Assume there is no heat exchange with the surroundings. For water, the specific heat is 4190 J/kg · K and the heat of fusion is 3.34 × 105 J/kg. The specific heat for the metal is closest to
A) 3850 J/kg ∙ K.
B) 2730 J/kg ∙ K.
C) 4450 J/kg ∙ K.
D) 4950 J/kg ∙ K.
E) 5450 J/kg ∙ K.
Correct Answer:
Verified
Q40: Thermal expansion: Suppose that a steel bridge,
Q41: Calorimetry: Two experimental runs are performed to
Q42: Calorimetry: A copper cylinder with a mass
Q43: Conduction of heat: What is the steady
Q44: Calorimetry: How many grams of ice at
Q46: Calorimetry: A 400-g piece of metal at
Q47: Calorimetry: Two experimental runs are performed to
Q48: Conduction of heat: Under steady state conditions,
Q49: Phase changes: If 2.0 g of water
Q50: Calorimetry: A person pours 330 g of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents