Calorimetry: How many grams of ice at - 13°C must be added to 711 grams of water that is initially at a temperature of 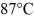 to produce water at a final temperature of
to produce water at a final temperature of 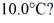 Assume that no heat is lost to the surroundings and that the container has negligible mass. The specific heat of liquid water is 4190 J/kg · °C and of ice is 2050 J/kg · °C. For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg. The normal boiling point is 100°C and the heat of vaporization is 2.26 × 106 J/kg.
Assume that no heat is lost to the surroundings and that the container has negligible mass. The specific heat of liquid water is 4190 J/kg · °C and of ice is 2050 J/kg · °C. For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg. The normal boiling point is 100°C and the heat of vaporization is 2.26 × 106 J/kg.
Correct Answer:
Verified
Q39: Quantity of heat: It is necessary to
Q40: Thermal expansion: Suppose that a steel bridge,
Q41: Calorimetry: Two experimental runs are performed to
Q42: Calorimetry: A copper cylinder with a mass
Q43: Conduction of heat: What is the steady
Q45: Calorimetry: A 200-g metal container, insulated on
Q46: Calorimetry: A 400-g piece of metal at
Q47: Calorimetry: Two experimental runs are performed to
Q48: Conduction of heat: Under steady state conditions,
Q49: Phase changes: If 2.0 g of water
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents