Ideal gas law: A sealed 89- 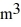 tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 350 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The initial pressure of the gas is closest to
tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 350 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The initial pressure of the gas is closest to
A) 0.15 MPa.
B) 0.17 MPa.
C) 0.19 MPa.
D) 0.13 MPa.
E) 0.11 MPa.
Correct Answer:
Verified
Q57: Phase changes: A 406.0 kg copper bar
Q58: Phase changes: A substance has a melting
Q59: Phase changes: A substance has a melting
Q60: Phase changes: If you add 700 kJ
Q61: Radiation: A spherical object 25.0 cm in
Q63: Radiation: A cube at 100.0°C radiates heat
Q64: Radiation: Betelgeuse is a red supergiant star
Q65: Radiation: A cube at 100°C radiates heat
Q66: Conduction of heat: The walls of an
Q67: Ideal gas law: A vertical tube that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents