Molecular speeds: What is the total translational kinetic energy in a test chamber filled with nitrogen (N2) at 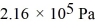 and
and 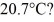 The dimensions of the chamber are
The dimensions of the chamber are 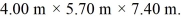 The ATOMIC weight of nitrogen is 28.0 g/mol, Avogadro's number is 6.022 × 1023 molecules/mol and the Boltzmann constant is 1.38 × 10-23 J/K.
The ATOMIC weight of nitrogen is 28.0 g/mol, Avogadro's number is 6.022 × 1023 molecules/mol and the Boltzmann constant is 1.38 × 10-23 J/K.
Correct Answer:
Verified
Q91: Ideal gas law: An ideal gas is
Q92: Molecular speeds: The root-mean-square speed (thermal speed)
Q93: Ideal gas law: 3.00 moles of an
Q94: Ideal gas law: A 3.2-L volume of
Q95: Ideal gas law: Sometimes an experiment requires
Q97: Ideal gas law: The figure shows a
Q98: Ideal gas law: What is the mass
Q99: Ideal gas law: A weather balloon contains
Q100: Molecular speeds: A 5.0-liter gas tank holds
Q101: Molecular speeds: At what temperature would the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents