Heat engines: A heat engine performs the reversible cycle abca with 9.0 moles of an ideal gas, as shown in the figure. Path ca is an adiabatic process. The temperatures at points a and b are 300 K and 500 K, respectively. The volume at point c is 0.20 m3. The adiabatic constant of the gas is 1.60. The thermal efficiency of this engine is closest to 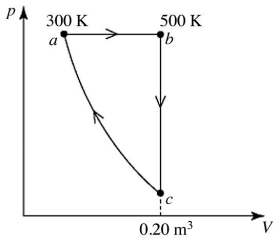
A) 0.070.
B) 0.10.
C) 0.13.
D) 0.16.
E) 0.19.
Correct Answer:
Verified
Q1: Carnot engine: An engine manufacturer makes the
Q2: Heat engines: Is it possible to transfer
Q3: Entropy: According to the second law of
Q4: Heat engines: Is it possible to transfer
Q6: Heat engines: A nuclear fission power plant
Q7: Entropy: A hot piece of iron is
Q8: Heat engines: A certain engine extracts 1300
Q9: Entropy: The entropy of an isolated system
Q10: Refrigerators: A refrigerator removes heat from the
Q11: Heat engines: A heat engine takes 2.0
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents