Molar heat capacities: A monatomic ideal gas undergoes an isothermal expansion at 300 K, as the volume increased from 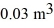 to
to 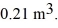 The final pressure of the gas is
The final pressure of the gas is 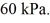 The ideal gas constant is R = 8.314 J/mol ∙ K. The change in the internal (thermal) energy of the gas is closest to
The ideal gas constant is R = 8.314 J/mol ∙ K. The change in the internal (thermal) energy of the gas is closest to
A) 0.00 kJ.
B) 12 kJ.
C) 25 kJ.
D) -12 kJ.
E) -25 kJ.
Correct Answer:
Verified
Q32: Molar heat capacities: 3.0 moles of an
Q33: First law of thermodynamics: In an isochoric
Q34: Molar heat capacities: The temperature of an
Q35: Molar heat capacities: The temperature of an
Q36: Molar heat capacities: A monatomic ideal gas
Q38: First law of thermodynamics: During an adiabatic
Q39: First law of thermodynamics: During an isothermal
Q40: Molar heat capacities: An ideal monatomic gas
Q41: First law of thermodynamics: A cylinder contains
Q42: First law of thermodynamics: An ideal gas
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents