Molar heat capacities: An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram. The initial pressure is 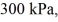 the initial volume is
the initial volume is 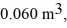 and the initial temperature is
and the initial temperature is 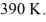 The ideal gas constant is R = 8.314 J/mol ∙ K. The final pressure is
The ideal gas constant is R = 8.314 J/mol ∙ K. The final pressure is 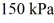 and the final temperature is
and the final temperature is 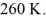 The work done by the gas is closest to
The work done by the gas is closest to
A) 4500 J.
B) 2300 J.
C) 3400 J.
D) 5600 J.
E) 6800 J.
Correct Answer:
Verified
Q20: Type of thermodynamic processes: The figure shows
Q21: First law of thermodynamics: In an isochoric
Q22: Molar heat capacities: An adiabatic compression is
Q23: First law of thermodynamics: During an isothermal
Q24: Molar heat capacities: An expansion process on
Q26: Molar heat capacities: An ideal gas with
Q27: Molar heat capacities: A quantity of ideal
Q28: Molar heat capacities: A certain amount of
Q29: Molar heat capacities: A compression, at a
Q30: First law of thermodynamics: The gas in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents