An analytical chemist wanted to use electrolysis to determine the number of moles of cupric ions in a given volume of solution. The solution was partitioned into n = 30 portions of 0.2 milliliter each. Each of the n = 30 portions was tested. The average number of moles of cupric ions for the n = 30 portions was found to be 0.185 mole; the standard deviation was 0.015 mole. Calculate the following intervals: 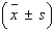 = ______________
= ______________ 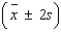 = ______________
= ______________ 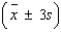 = ______________
= ______________
Describe the distribution of the measurements for the n = 30 portions of the solution using Tchebysheff's Theorem.
________________________________________________________
Describe the distribution of the measurements for the n = 30 portions of the solution using the Empirical Rule.
________________________________________________________
Suppose the chemist had used only n = 5 portions of the solution for the experiment and obtained the readings 0.18, 0.21, 0.20, 0.22, and 0.18. Would the Empirical Rule be suitable for describing the n = 5 measurements?
______________
Explain.
________________________________________________________
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q83: A data sample has a mean of
Q84: The times required to service customers' cars
Q85: If the sample mean is much larger
Q86: Two classes, one with 15 students and
Q87: Numerical descriptive measures computed from sample measurements
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents