Consider a sample of water in a closed, rigid container. Which of the following will indicate that the given physical process has reached equilibrium? 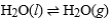
A) The mass of the container remains constant.
B) The vapor pressure in the container remains constant.
C) The volume of water in the container increases from its original level.
D) All of these indicate that the reaction has reached equilibrium.
Correct Answer:
Verified
Q37: In a particular chemical reaction, four bonds
Q38: Solid sodium chloride and solid silver nitrate
Q39: In a particular chemical reaction, two bonds
Q40: If a chemist wishes to react a
Q41: What is the effect of increasing the
Q43: Which of the following is an example
Q44: If a particular reactant is involved in
Q45: Which of the following statements is true
Q46: What is the effect of decreasing the
Q47: A catalyst speeds up a chemical reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents