Acetic acid is the active ingredient in vinegar. In a solution of acetic acid, the following equilibrium is established. 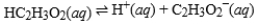 The equilibrium constant for this reaction is 1.5 × 10-5. What is the equilibrium constant for the following reaction?
The equilibrium constant for this reaction is 1.5 × 10-5. What is the equilibrium constant for the following reaction? 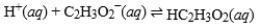
A) 1.8 × 10-5
B) 4.2 × 10-3
C) 6.6 × 104
D) 3.1 × 109
Correct Answer:
Verified
Q71: In an equilibrium constant expression, what does
Q72: For the reaction Q73: For the reaction Q74: Which of the following will change the Q75: Suppose the equilibrium constant for the chemical Q77: Consider a sample of water in a Q78: For the reaction Q79: Which of the following will change the Q80: For the reaction Q81: What is the effect of adding CH3COOC2H5(g)to Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()
![]()

