When the state of a substance changes from A to B as represented by the given models, which of the following statements is true? 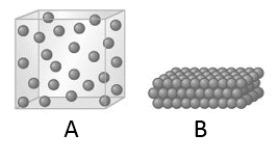
A) The velocity of the molecules increases.
B) The strength of the intermolecular forces increases.
C) The kinetic energy of the molecules decreases.
D) The strength of the intermolecular force increases and the kinetic energy of the molecules decreases.
Correct Answer:
Verified
Q5: Which physical state of matter is represented
Q6: Which of the following directly determine the
Q7: Which of the following is the SI
Q8: Which of the following instruments is used
Q9: Which of the following laws relates the
Q11: Which of the following is a mathematical
Q12: At constant pressure, the temperature of a
Q13: The temperature of a substance is decreased
Q14: What is the average pressure of the
Q15: Which of the following laws relates the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents