Examine the following image representing the structure of a compound. 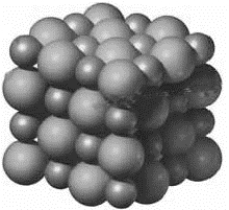 If the smaller spheres represent K+ and the larger spheres represent Cl−, then what is the name of this compound?
If the smaller spheres represent K+ and the larger spheres represent Cl−, then what is the name of this compound?
A) potassium dichloride
B) dipotassium chloride
C) monopotassium dichloride
D) potassium chloride
Correct Answer:
Verified
Q122: Which of the following molecules is not
Q123: The following represents carbonic acid, an important
Q124: The following represents carbonic acid, an important
Q125: Which of the following molecules is nonpolar?
A)
Q126: Which of the following is true of
Q128: Examine the following image representing the structure
Q129: Which of the following molecules is polar?
A)
Q130: The following represents carbonic acid, an important
Q131: Which of the following molecules is not
Q132: Which of the following molecules is polar?
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents