Consider the following structure. 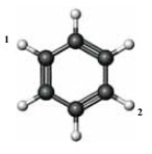 If a chlorine atom is substituted for each of the labeled hydrogen atoms, which of the following is true of the resulting compound?
If a chlorine atom is substituted for each of the labeled hydrogen atoms, which of the following is true of the resulting compound?
A) The compound can have cis-trans isomers.
B) The compound will have substituents in its meta positions.
C) The compound will have substituents bonded to its 1,4 positions.
D) The compounds will have substituents in its meta positions and bonded to its 1,4 positions.
Correct Answer:
Verified
Q156: A compound contains a benzene ring with
Q157: Which of the following structures represents m-nitrobenzoic
Q158: What is the correct IUPAC name of
Q159: Which of the following structures represents 2,4,6-tribromophenol?
A)
Q160: Which reagent is used to convert nitrobenzene
Q162: Which of the following products is obtained
Q163: How should a benzene ring with a
Q164: By which of the following reactions is
Q165: Consider the following structure. Q166: Which of the following is a common![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents