Fluoxymesterone is an anabolic steroid with strong androgenic properties, which has been used for the treatment of hormone-sensitive breast tumors in women. It is also misused as an aggression enhancer in boxing and martial arts competitions. Since the half-life of this hormone in the human body is more than 9h, it can be easily detected in a doping sample taken after the competition. Compare the in-vivo physical properties of both hormones. Which statements below are correct? 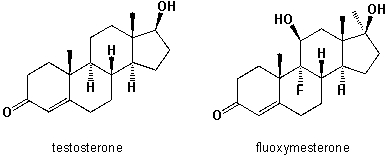 I) The exchange of a hydrogen atom vs. a fluorine atom does not significantly change the water-solubility, because the C-F bond is short and an organically bound fluorine-atom is almost as small as a carbon-bonded hydrogen atom.
I) The exchange of a hydrogen atom vs. a fluorine atom does not significantly change the water-solubility, because the C-F bond is short and an organically bound fluorine-atom is almost as small as a carbon-bonded hydrogen atom.
II) The exchange of a hydrogen vs. a fluorine atom does significantly change the water-solubility, because the C-F bond is non-polar covalent, whereas C-H is polar covalent.
III) The introduction of a second hydroxy-group at the C-ring of fluoxymesterone increases the water-solubility.
IV) Fluoxymesterone is less water soluble than testosterone, because it possesses 19 carbon atoms, compared to 19 of testosterone.
A) I and II
B) II and III
C) I and III
D) I, III, and IV
Correct Answer:
Verified
Q22: What do all prostaglandins have in common?
I.
Q23: Order the four vitamins according to increasing
Q24: Complete the reaction below by providing the
Q25: The melting point order of the following
Q26: Complete the structure of the alkylbenzene sulfonate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents