Only the S-enantiomer of naproxen inhibits the COX-1 and COX-2 enzymes, the R-enantiomer is a strong liver toxin. Therefore, only enantiomerically pure naproxen is sold in US pharmacies. Unfortunately, the classic synthesis leads to a racemic mixture. Which base do you use to separate the two enantiomers by recrystallization? 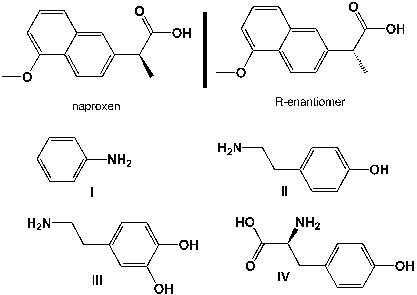
A) I
B) II
C) III
D) IV
Correct Answer:
Verified
Q29: Which acid/base salts differ in their physical
Q30: An ether solution containing all of the
Q31: The reagents that complete the following reaction
Q32: Which acid is strongest? Q33: Which conditions will convert pentanoic acid to Q35: An ether solution containing all of the Q36: The IUPAC name of the following structure Q37: The IUPAC name of the following structure Q38: The following compound is prepared by Fischer Q39: The amino acid tryptophan undergoes enzymatic decarboxylation![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents