A compound has the molecular formula C5H10O, and strong absorptions at 1100 and 3350 cm-1. Which is the most likely structure for the compound? 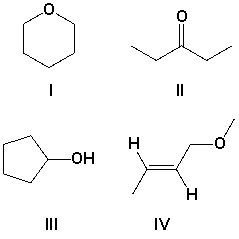
A) I
B) II
C) III
D) IV
Correct Answer:
Verified
Q2: Very prominent peaks in the IR spectrum
Q3: Which is the approximate energy range for
Q4: How many principal vibrations does aspartame have?
Q5: Which is the order of increasing bond
Q6: Which region in the IR spectrum could
Q8: Which is the wavelength (
Q9: The broadening of the stretching vibration peak
Q10: Which is the index of hydrogen deficiency
Q11: Which is the approximate energy range for
Q12: Prominent peaks in the IR spectrum are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents