When the following compounds are dissolved in water at the same concentrations, which one will have the lowest pH (i.e., most acidic) ?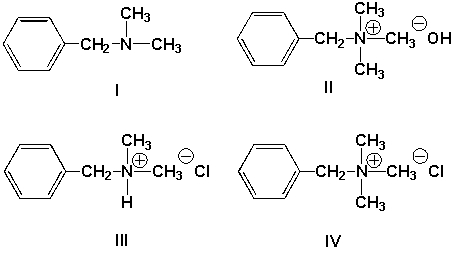
A) I
B) II
C) III
D) IV
Correct Answer:
Verified
Q24: The order of increasing basicity of the
Q25: The starting material needed to complete the
Q26: The name of the following compound is
Q27: The major product of the following reaction
Q28: At pH 5.0, the ratio of morpholine
Q30: Which reactions will proceed predominantly to products
Q31: The following mixture was extracted with 1
Q32: The starting material needed to complete the
Q33: Which is the product of the following
Q34: The starting material and reactants needed to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents