Using the VSEPR model, predict which species have bond angles of about 109°.
HINT: Assume that the charges are correct. Add the missing lone pairs before applying the VSEPR theory!
A) I, III, IV 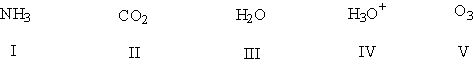
B) II, III, V
C) I, IV
D) III, IV, V
Correct Answer:
Verified
Q1: A neutral carbon has how many valence
Q2: A neutral nitrogen has how many valence
Q3: Which is the correct Lewis structure for
Q4: A neutral oxygen has how many valence
Q5: Which Lewis structures are correct?
HINT: Perform a
Q7: Which is the electronic configuration that describes
Q8: Which functional groups have correct Lewis structures?
Q9: Which atom is described by the electronic
Q10: Which statement about orbitals is false?
A) Orbitals
Q11: Which is the electronic configuration that describes
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents