What are the correct orbital hybridizations for carbon in the following species?
HINT: Add a lone pair if the charge of the molecule suggests it!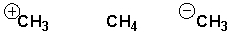
A) CH3+ is sp-hybridized
B) CH3- is sp2-hybridized
C) CH4 is sp2-hybridized
D) CH3- is sp3-hybridized
Correct Answer:
Verified
Q29: Which compounds contain both covalent and ionic
Q30: Which of these molecules are polar?
HINT: look
Q31: Which of these molecules are polar?
HINT: look
Q32: The spins of two electrons in the
Q33: Which of the following are pairs of
Q35: Which statement about contributing structures is false?
A)
Q36: Which of the three molecules testosterone, methadone
Q37: Which of the three molecules (testosterone, methadone
Q38: Which of the following compounds is a
Q39: Which of the three molecules testosterone, methadone
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents