Compound X has a molecular formula C8H14 and reacts with H2/Pt to give compound Y, C8H16. Which is compound X? 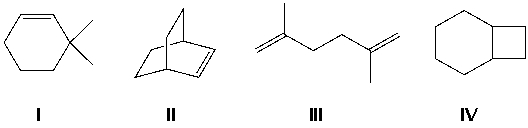
A) I
B) II
C) III
D) IV
Correct Answer:
Verified
Q59: Each shell can hold two electrons.
Q60: Which is the major product from the
Q61: Arrange these carbocations in order of increasing
Q62: Which reagents react with an alkene by
Q63: Which are the major products from the
Q65: Which compound has the most exothermic (most
Q66: Which reagents react with 2-methyl-1-hexene in a
Q67: Which energy diagram represents the reaction of
Q68: Which is the major product from the
Q69: Which reagents react with an alkene by
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents