The activation energies of the reaction represented by the following reaction energy diagram are noted as I and II. 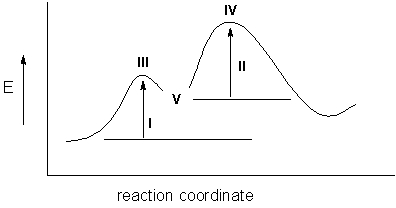
Correct Answer:
Verified
Q82: The product of the reaction of cyclohexene
Q83: The rate-determining step of the reaction represented
Q84: Which diagram represents the slowest reaction?
Q85: Which is the intermediate formed in the
Q86: Which alkene has the highest rate of
Q88: Order the four compounds according to increasing
Q89: The following alkenes are listed in decreasing
Q90: Which statements describes an intermediate?
A) cannot be
Q91: 1-Cyclohexyl-1-butyne reacts with molecular hydrogen under pressure
Q92: The reaction represented by the following energy
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents