Below is a representation of a sample of gas with molecules having the formula XO2. Which element could NOT be represented by X? 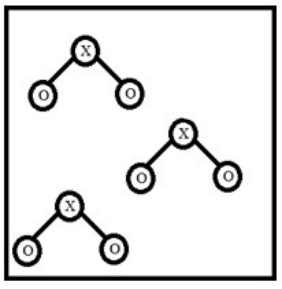
A) N
B) S
C) C
D) O
E) Cl
Correct Answer:
Verified
Q17: The acidity of oxides of main group
Q43: The chlor-alkali process produces chlorine, Cl2(g), in
Q45: Alkali metal hydrides are very reactive with
Q46: P4O6 and P4O10 are allotropes of phosphorus.
Q91: Which is true concerning the difference between
Q92: Which is NOT a hydride?
A) 
Q93: Which reaction represents the disproportionation of hydrogen
Q94: Nonmetals are more electropositive than metals.
Q98: Hydrogen is placed at the top of
Q101: _ _ are formed when molecular hydrogen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents