Which compound, if any, will not be optically active?
A) 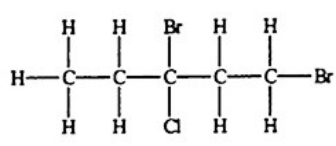
B) 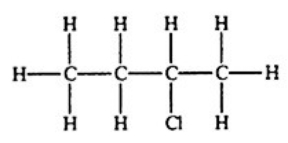
C) 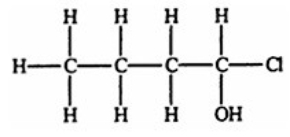
D) 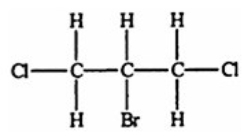
E) All of the structures are optically active.
Correct Answer:
Verified
Q16: How many structural isomers are there of
Q62: What is the molecular formula for the
Q63: What is the name of the following
Q64: What is the name of the following
Q65: The two molecules represented below are examples
Q66: Which type of isomerism is described as
Q68: Which has resonance structures?
A) CH4
B) CH3CH2COO−
C) CH3CH2CH3
D)
Q69: What is the molecular formula for the
Q71: The two molecules represented below are examples
Q72: Which of these species are structural isomers
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents