Calculate the energy released in joules when one mole of polonium-214 decays according to the following equation. 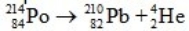
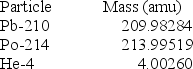 (1 kg = 6.022 × 1026 amu; NA = 6.022 × 1023 mol-1;
(1 kg = 6.022 × 1026 amu; NA = 6.022 × 1023 mol-1;
C = 2.99792458 × 108 m/s)
A) 8.78 × 1014 J/mol
B) 7.2 × 1014 J/mol
C) 8.76 × 1011 J/mol
D) -9.75 × 10-3 J/mol
E) 1.46 × 10-9 J/mol
Correct Answer:
Verified
Q21: What fraction of radioactive atoms remains in
Q22: The radiochemist, Will I. Glow, studied thorium-232
Q23: A mass of 6.02 × 1026 amu
Q25: What is the nuclear binding energy per
Q27: Iodine-131, t1/2 = 8.0 days, is used
Q28: What is the nuclear binding energy per
Q29: The nuclear binding energy per nucleon is
Q37: An isotope with a high value of
Q48: Charcoal found under a stone at Stonehenge,
Q60: Polonium-208 is an alpha emitter with a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents