Determine how much energy is released when thorium-230 decays according to the following equation. 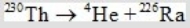
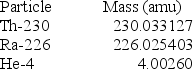 (1 kg = 6.022 × 1026 amu; NA = 6.022 × 1023 mol-1;
(1 kg = 6.022 × 1026 amu; NA = 6.022 × 1023 mol-1;
C = 2.99792458 × 108 m/s)
A) 3.98 × 109 kJ/mol
B) 4.61 × 108 kJ/mol
C) 7.20 × 1011 kJ/mol
D) 4.90 × 109 kJ/mol
E) 7.15 × 1011 kJ/mol
Correct Answer:
Verified
Q22: 1 joule equals
A) 1 kg m
B) 1
Q33: What is the energy equivalent of 1
Q34: What is the name for the difference
Q35: A rock contains 0.37 mg of Pb-206
Q36: The 14C activity of some ancient Peruvian
Q40: What is the nuclear binding energy per
Q42: In the following reaction, identify X.
Q43: The half-life of 14C is 5730 yr.
Q47: The radioisotope potassium-40 decays to argon-40 by
Q54: Cobalt-60 is a beta emitter with a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents