A cell can be prepared from copper and tin. What is the E°cell for the galvanic cell that forms from the following half-reactions? 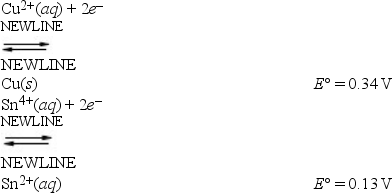
A) 0.47 V
B) 0.21 V
C) -0.21 V
D) -0.47 V
E) 0.42 V
Correct Answer:
Verified
Q31: What is meant by SHE?
A) Shared half
Q32: What is E°cell for a galvanic cell
Q33: What is the purpose of a salt
Q34: What is E°cell for the following reaction?
Q35: Which is the correct cell notation for
Q37: What is the name given to the
Q38: What is E°cell for a galvanic cell
Q39: Based on the data presented below, which
Q40: A certain electrochemical cell has for its
Q41: If ΔG° of the following reaction is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents