What is the standard free-energy change for the following reaction at 25°C? (F = 96,500 C • mol-1) 2Al(s) + 3Sn4+(aq,1 M) → 2Al3+(aq,1 M) + 3Sn2+(aq,1 M) 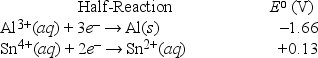
A) -1.79 kJ/mol
B) 1.79 kJ/mol
C) -1.04 × 103 kJ/mol
D) -3.45 × 102 kJ/mol
E) 5.18 × 102 kJ/mol
Correct Answer:
Verified
Q83: Which of the following is correct?
A) total
Q85: A current of 250. A flows for
Q86: Based on the following electrochemical cell, which
Q86: Which element is associated with the term
Q87: If the following electrochemical cell is constructed,
Q89: Based on the following electrochemical cell, what
Q90: The equilibrium constant for the reaction of
Q91: Which component of the following cell is
Q92: Which of the following elements could be
Q92: Based on the following electrochemical cell, what
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents