Which electrochemical cell pictured below corresponds to the following cell diagram? Cr(s) | Cr3+(aq, 1.0 M) || Sn2+(aq, 1.0 M) | Sn(s)
A) 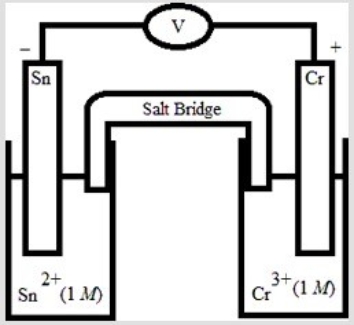
B) 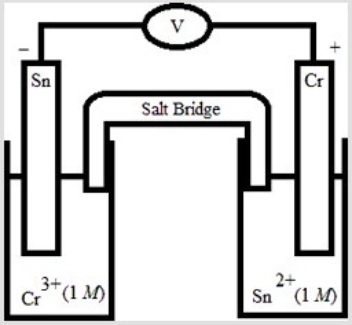
C) 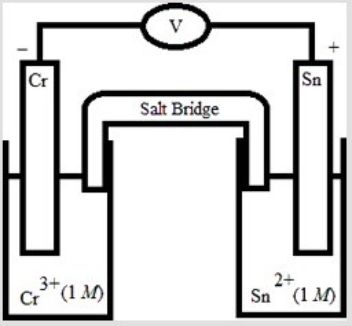
D) 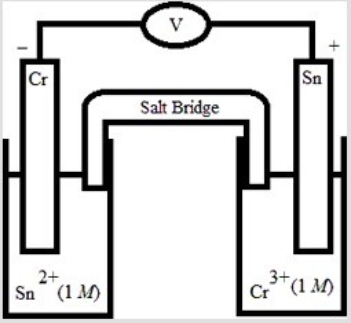
E) 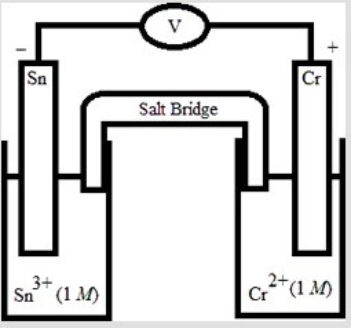
Correct Answer:
Verified
Q15: In a fuel cell, an external source
Q95: Two cells are connected in series, so
Q95: What product forms at the anode during
Q97: Given Cu2+(aq) + 2e- → Cu(s) E°
Q98: Aluminum does not corrode in the same
Q98: Which of the following elements can be
Q100: Which equation is correct?
A) Ecell = RT
Q101: Lithium-ion batteries can be recharged many times.
Q102: The Faraday constant represents the charge of
Q104: E > 0 and ΔG < 0
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents