Calculate ΔG° for the combustion of ethanol vapor, C2H5OH(g) , at 750.°C in oxygen to form carbon dioxide and water vapor. The following data are valid at 25°C: 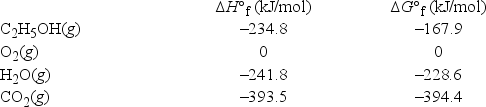
A) -1407 kJ/mol
B) -2151 kJ/mol
C) -1307 kJ/mol
D) -4486 kJ/mol
E) -1377 kJ/mol
Correct Answer:
Verified
Q10: The higher the pressure of a gas
Q46: Which species will have the greatest absolute
Q62: The absolute standard entropy of atom X(g)
Q63: For the following process, which is true?
Q64: For the process C6H6(l) <-----> C6H6(s) at
Q65: In 1774 Joseph Priestly prepared oxygen by
Q66: Which statement is correct?
A) Oxygen is formed
Q68: Is the following process spontaneous? 
Q69: Below are energy level diagrams for two
Q72: Which statement is correct?
A) Reaction of ADP
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents