Consider the figure below which shows ΔG° for a chemical process plotted against absolute temperature. From this plot, it is reasonable to conclude that: 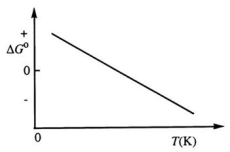
A) ΔH° > 0, ΔS° > 0
B) ΔH° > 0, ΔS° < 0
C) ΔH° < 0, ΔS° > 0
D) ΔH° < 0, ΔS° < 0
E) None of these choices is correct.
Correct Answer:
Verified
Q56: Elemental boron can be formed by the
Q57: Sulfuryl dichloride may be formed from the
Q58: For a chemical reaction to be spontaneous
Q59: HI has a normal boiling point of
Q60: Hydrogen sulfide decomposes according to the following
Q62: The absolute standard entropy of atom X(g)
Q63: For the following process, which is true?
Q64: For the process C6H6(l) <-----> C6H6(s) at
Q65: In 1774 Joseph Priestly prepared oxygen by
Q66: Which statement is correct?
A) Oxygen is formed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents