Consider the figure below which shows ΔG° for a chemical process plotted against absolute temperature. From this plot, it is reasonable to conclude that: 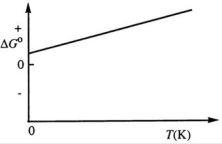
A) ΔH° > 0, ΔS° > 0
B) ΔH° > 0, ΔS° < 0
C) ΔH° < 0, ΔS° > 0
D) ΔH° < 0, ΔS° < 0
E) None of these choices is correct.
Correct Answer:
Verified
Q10: The higher the pressure of a gas
Q48: Which species will have the lowest absolute
Q72: Which statement is correct?
A) Reaction of ADP
Q74: The reaction of methane with water to
Q75: As the molar mass of a compound
Q77: Which of the following substances has the
Q78: At temperatures below 273 K, it is
Q79: The heat of vaporization of 1-pentanol is
Q80: Consider the figure below which shows ΔG°
Q81: The entropy of vaporization of a compound
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents