A 20.0-mL sample of 0.30 M HClO was titrated with 0.30 M NaOH. The following data were collected during the titration. Determine to Ka for HClO. 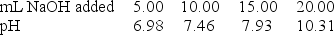
A) 1.1 × 10-7
B) 3.5 × 10-8
C) 1.2 × 10-8
D) 4.9 × 10-11
E) None of the answers is correct.
Correct Answer:
Verified
Q33: Methyl red is a common acid-base indicator.It
Q38: What is the pH of a solution
Q39: Which is the best acid to prepare
Q40: Which of the following is an exact
Q41: A 25.0-mL sample of 1.00 M NH3
Q42: When a weak acid is titrated with
Q44: Calculate the pH at the equivalence point
Q45: The solubility of lead(II) iodide is 0.064
Q46: When a strong acid is titrated with
Q48: For PbCl2 (Ksp = 2.4 × 10-4),
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents