Which one of the following is the best representation of the titration curve which will be obtained in the titration of a weak diprotic acid H2A (0.10 M) with a strong base of the same concentration?
A) 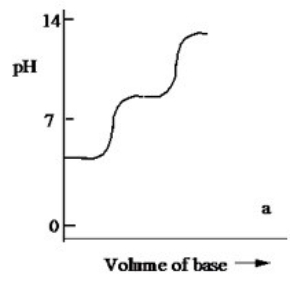
B) 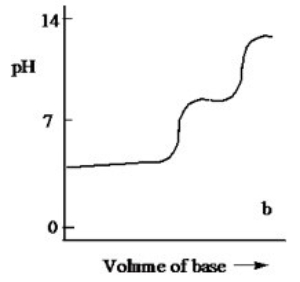
C) 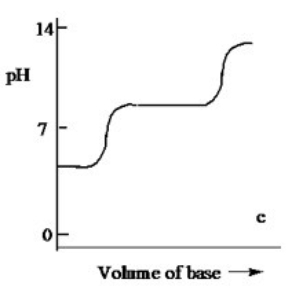
D) 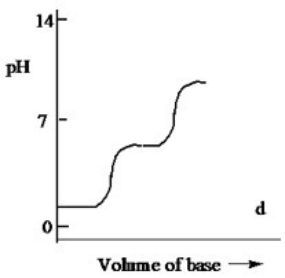
E) 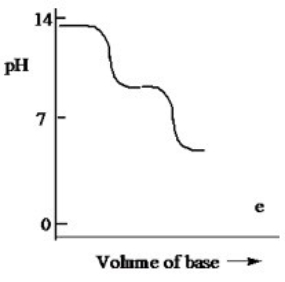
Correct Answer:
Verified
Q29: Calculate the pH of the solution resulting
Q50: When a strong acid is titrated with
Q51: The indicator propyl red has Ka =
Q52: A 10.0-mL sample of 0.75 M CH3CH2COOH
Q53: Which of the following is correct?
A) Solubility
Q54: Which indicator would be the best to
Q56: Which is included in the Ksp expression?
Q59: A 25.0-mL sample of 0.10 M C2H5NH2
Q60: 40.0 ml of an acetic acid solution
Q73: A 35.0-mL sample of 0.20 M LiOH
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents